Advanced MSI-H/dMMR Colorectal Cancer (CRC)
KEYTRUDA is indicated for the treatment of patients with unresectable or metastatic MSI-H or dMMR colorectal cancer (CRC) as determined by an FDA-approved test.
KEYTRUDA is indicated for the treatment of patients with unresectable or metastatic MSI-H or dMMR colorectal cancer (CRC) as determined by an FDA-approved test.
| KEYTRUDA (n=153) | Chemotherapy (n=154) |
|---|---|
HRh=0.60; 95% CI, 0.45–0.80; Pi=0.0004 | |
Events observed54%(n=82/153) | Events observed73%(n=113/154) |
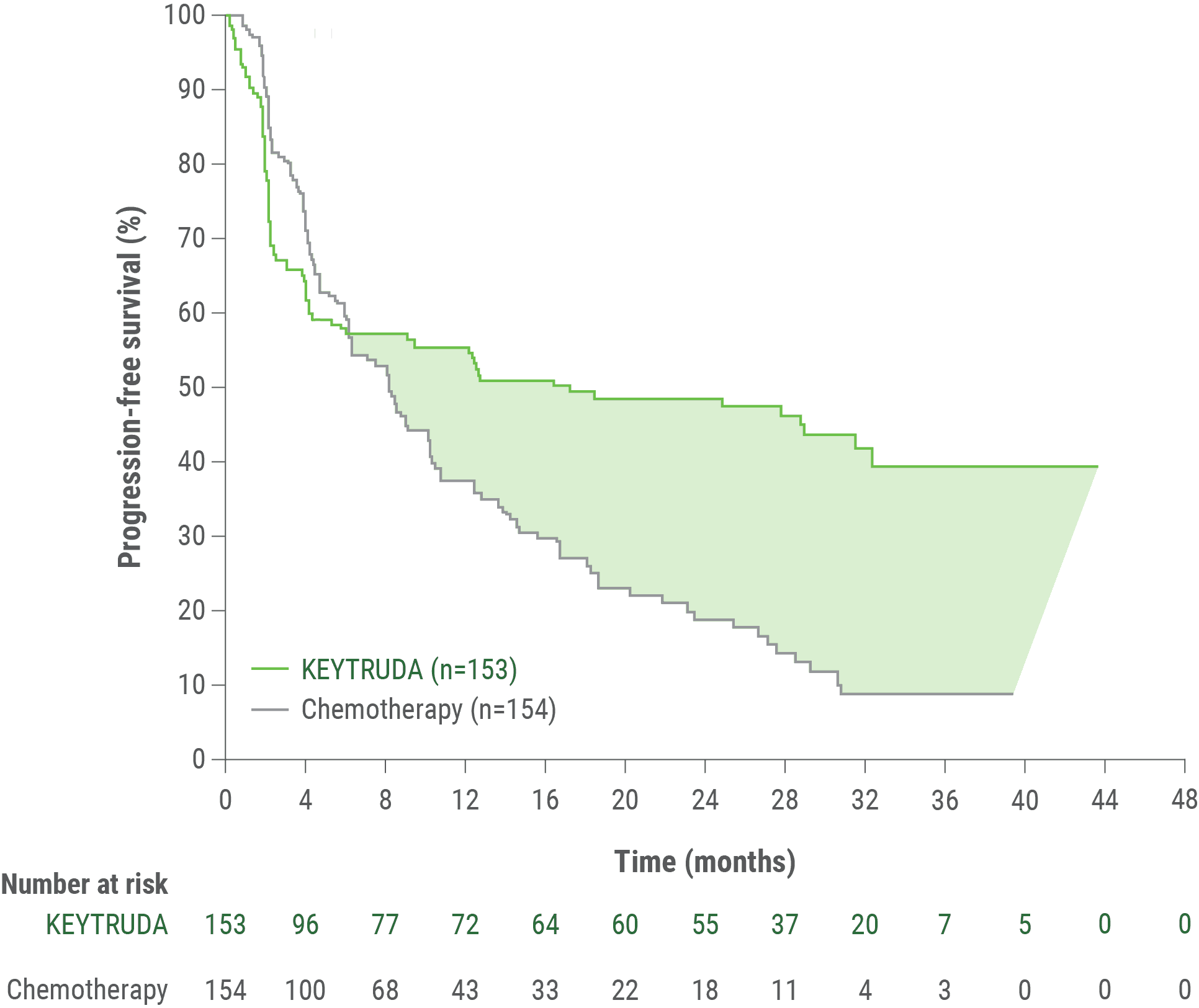
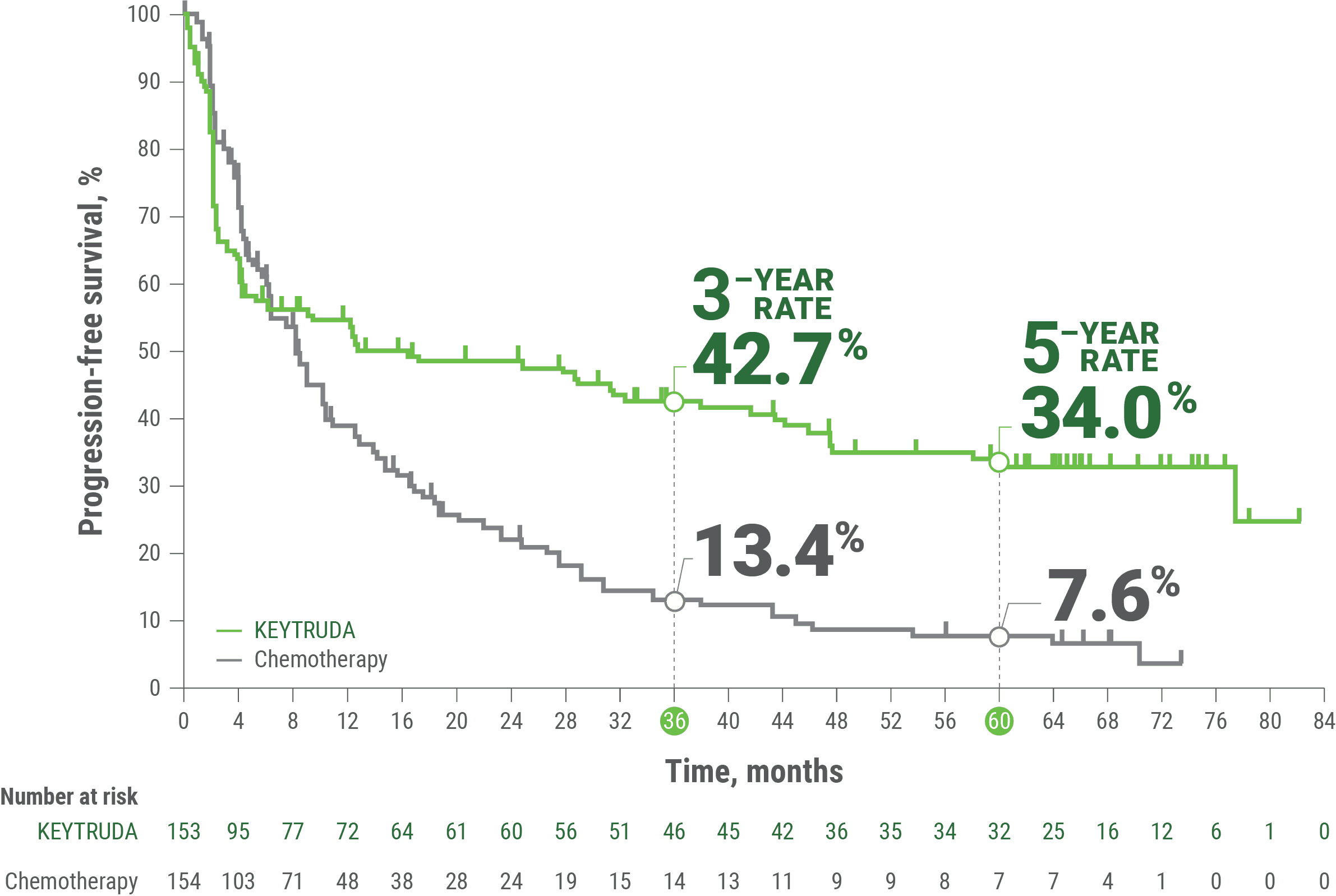
Median PFS16.5 months(95% CI, 5.4–32.4) | Median PFS8.2 months(95% CI, 6.1–10.2) |
| KEYTRUDA (n=153) | Chemotherapy (n=154) |
|---|---|
HRk=0.74; 95% CI, 0.53–1.03; Pl=0.0718 | |
Median OSNot reached (95% CI 49.2, Not reached) | Median OS36.7 months (95% CI 27.6, Not reached) |
Events Observed41%(n=62/153) | Events Observed51%(n=78/154) |
LIMITATIONS: The 5-year follow-up of KN-177 was a post hoc, exploratory analysis. No formal statistical testing was planned for this analysis and, therefore, no statistical conclusions can be drawn.
| KEYTRUDA (n=153) | Chemotherapy (n=154) |
|---|---|
Events observed61.4%(n=94/153) | Events observed79.2%(n=122/154) |
Median PFS16.5 months(95% CI, 5.4-38.1) | Median PFS8.2 months(95% CI, 6.2-10.3) |
| KEYTRUDA | Chemotherapy |
|---|---|
Median DORoNot reached(range: 2.3+q to 41.4+q months) | Median DORo10.6 months(range: 2.8 to 37.5+q months) |
| KEYTRUDA | Chemotherapy |
|---|---|
Median DOR75.4 months(range: 2.3+q to 80.1+q) | Median DOR10.6 months(range: 2.8 to 71.5+q months) |
The efficacy of KEYTRUDA was investigated in KEYNOTE-177, a multicenter, randomized, open-label, active-controlled trial that enrolled 307 patients with previously untreated unresectable or metastatic MSI-H or dMMR CRC. MSI or MMR tumor status was determined locally using polymerase chain reaction (PCR) or immunohistochemistry (IHC), respectively. Patients with autoimmune disease or a medical condition that required immunosuppression were ineligible. Patients were randomized (1:1) to receive KEYTRUDA 200 mg intravenously every 3 weeks or investigator’s choice of the following chemotherapy regimens given intravenously every 2 weeks: mFOLFOX6 (oxaliplatin, leucovorin, and FU) or mFOLFOX6 in combination with either bevacizumab or cetuximab: Oxaliplatin 85 mg/m2, leucovorin 400 mg/m2 (or levoleucovorin 200 mg/m2), and FU 400 mg/m2 bolus on Day 1, then FU 2400 mg/m2 over 46–48 hours. Bevacizumab 5 mg/kg on Day 1 or cetuximab 400 mg/m2 on first infusion, then 250 mg/m2 weekly; FOLFIRI (irinotecan, leucovorin, and FU) or FOLFIRI in combination with either bevacizumab or cetuximab: Irinotecan 180 mg/m2, leucovorin 400 mg/m2 (or levoleucovorin 200 mg/m2), and FU 400 mg/m2 bolus on Day 1, then FU 2400 mg/m2 over 46–48 hours. Bevacizumab 5 mg/kg on Day 1 or cetuximab 400 mg/m2 on first infusion, then 250 mg/m2 weekly. Treatment with KEYTRUDA or chemotherapy continued until RECIST v1.1-defined progression of disease as determined by the investigator or unacceptable toxicity. Patients treated with KEYTRUDA without disease progression could be treated for up to 24 months. Assessment of tumor status was performed every 9 weeks. Patients randomized to chemotherapy were offered KEYTRUDA at the time of disease progression.
The median duration of exposure to KEYTRUDA was 11.1 months (range: 1 day to 30.6 months).
The main efficacy outcome measures were PFS as assessed by BICR according to RECIST v1.1, modified to follow a maximum of 10 target lesions and a maximum of 5 target lesions per organ, and OS.
Additional efficacy outcome measures were ORR and DOR.
Patients randomized to chemotherapy were offered KEYTRUDA at the time of disease progression.
Baseline Characteristics:
Among 154 patients randomized to receive chemotherapy, 143 received chemotherapy per protocol
KEYTRUDA is indicated for the treatment of patients with unresectable or metastatic MSI-H or dMMR colorectal cancer (CRC) as determined by an FDA-approved test.
KEYTRUDA is indicated for the treatment of patients with unresectable or metastatic melanoma.
KEYTRUDA is indicated for the adjuvant treatment of adult and pediatric (12 years and older) patients with stage IIB, IIC, or III melanoma following complete resection.
KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of patients with metastatic nonsquamous non–small cell lung cancer (NSCLC), with no epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) genomic tumor aberrations.
KEYTRUDA, in combination with carboplatin and either paclitaxel or paclitaxel protein‑bound, is indicated for the first‑line treatment of patients with metastatic squamous non–small cell lung cancer (NSCLC).
KEYTRUDA, as a single agent, is indicated for the first-line treatment of patients with non–small cell lung cancer (NSCLC) expressing programmed death ligand 1 (PD-L1) [tumor proportion score (TPS) ≥1%] as determined by an FDA-approved test, with no epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) genomic tumor aberrations, and is:
KEYTRUDA, as a single agent, is indicated for the treatment of patients with metastatic non–small cell lung cancer (NSCLC) whose tumors express programmed death ligand 1 (PD-L1) [tumor proportion score (TPS) ≥1%] as determined by an FDA-approved test, with disease progression on or after platinum-containing chemotherapy. Patients with epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving KEYTRUDA.
KEYTRUDA is indicated for the treatment of patients with resectable (tumors ≥4 cm or node positive) non–small cell lung cancer (NSCLC) in combination with platinum-containing chemotherapy as neoadjuvant treatment, and then continued as a single agent as adjuvant treatment after surgery.
KEYTRUDA, as a single agent, is indicated as adjuvant treatment following resection and platinum-based chemotherapy for adult patients with stage IB (T2a ≥4 cm), II, or IIIA non–small cell lung cancer (NSCLC).
KEYTRUDA is indicated for the treatment of adult patients with resectable locally advanced HNSCC whose tumors express PD-L1 [Combined Positive Score (CPS) ≥1] as determined by an FDA-approved test, as a single agent as neoadjuvant treatment, continued as adjuvant treatment in combination with radiotherapy (RT) with or without cisplatin and then as a single agent.
KEYTRUDA, in combination with platinum and fluorouracil (FU), is indicated for the first-line treatment of patients with metastatic or with unresectable, recurrent head and neck squamous cell carcinoma (HNSCC).
KEYTRUDA, as a single agent, is indicated for the first-line treatment of patients with metastatic or with unresectable, recurrent head and neck squamous cell carcinoma (HNSCC) whose tumors express programmed death ligand 1 (PD-L1) [combined positive score (CPS) ≥1] as determined by an FDA-approved test.
KEYTRUDA, as a single agent, is indicated for the treatment of patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) with disease progression on or after platinum-containing chemotherapy.
KEYTRUDA, in combination with enfortumab vedotin, is indicated for the treatment of adult patients with locally advanced (LA) or metastatic urothelial cancer (mUC).
KEYTRUDA, as a single agent, is indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for any platinum-containing chemotherapy.
KEYTRUDA, as a single agent, is indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who have disease progression during or following platinum-containing chemotherapy or within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.
KEYTRUDA, as a single agent, is indicated for the treatment of patients with Bacillus Calmette-Guerin (BCG)-unresponsive, high-risk, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors who are ineligible for or have elected not to undergo cystectomy.
KEYTRUDA is indicated for the treatment of adult and pediatric patients with unresectable or metastatic microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) solid tumors, as determined by an FDA-approved test, that have progressed following prior treatment and who have no satisfactory alternative treatment options.
KEYTRUDA is indicated for the treatment of patients with unresectable or metastatic MSI-H or dMMR colorectal cancer (CRC) as determined by an FDA-approved test.
KEYTRUDA, in combination with fluoropyrimidine- and platinum-containing chemotherapy, is indicated for the first-line treatment of adults with locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction (GEJ) adenocarcinoma whose tumors express PD-L1 (CPS ≥1) as determined by an FDA-approved test.
KEYTRUDA is indicated for the treatment of patients with locally advanced or metastatic esophageal or gastroesophageal junction (GEJ) (tumors with epicenter 1 to 5 centimeters above the GEJ) carcinoma that is not amenable to surgical resection or definitive chemoradiation either:
KEYTRUDA, in combination with chemoradiotherapy (CRT), is indicated for the treatment of patients with locally advanced cervical cancer involving the lower third of the vagina, with or without extension to pelvic sidewall, or hydronephrosis/non-functioning kidney, or spread to adjacent pelvic organs (FIGO 2014 Stage III-IVA).
KEYTRUDA, in combination with chemotherapy, with or without bevacizumab, is indicated for the treatment of patients with persistent, recurrent, or metastatic cervical cancer whose tumors express programmed death ligand 1 (PD-L1) [combined positive score (CPS) ≥1] as determined by an FDA-approved test.
KEYTRUDA, as a single agent, is indicated for the treatment of patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy whose tumors express programmed death ligand 1 (PD-L1) [combined positive score (CPS) ≥1] as determined by an FDA-approved test.
KEYTRUDA, in combination with gemcitabine and cisplatin, is indicated for the treatment of patients with locally advanced unresectable or metastatic biliary tract cancer (BTC).
KEYTRUDA is indicated for the treatment of adult and pediatric patients with recurrent locally advanced or metastatic Merkel cell carcinoma (MCC).
KEYTRUDA is indicated for the adjuvant treatment of patients with renal cell carcinoma (RCC) at intermediate-high or high risk of recurrence following nephrectomy, or following nephrectomy and resection of metastatic lesions.
KEYTRUDA, in combination with axitinib, is indicated for the first-line treatment of adult patients with advanced renal cell carcinoma (RCC).
KEYTRUDA, in combination with carboplatin and paclitaxel, followed by KEYTRUDA as a single agent, is indicated for the treatment of adult patients with primary advanced or recurrent endometrial carcinoma.
KEYTRUDA, as a single agent, is indicated for the treatment of adult patients with advanced endometrial carcinoma that is MSI-H or dMMR, as determined by an FDA-approved test, who have disease progression following prior systemic therapy in any setting and are not candidates for curative surgery or radiation.
KEYTRUDA is indicated for the treatment of patients with recurrent or metastatic cutaneous squamous cell carcinoma (cSCC) or locally advanced cSCC that is not curable by surgery or radiation.
KEYTRUDA is indicated for the treatment of patients with high-risk early-stage triple-negative breast cancer (TNBC) in combination with chemotherapy as neoadjuvant treatment, and then continued as a single agent as adjuvant treatment after surgery.
KEYTRUDA, in combination with chemotherapy, is indicated for the treatment of patients with locally recurrent unresectable or metastatic triple-negative breast cancer (TNBC) whose tumors express programmed death ligand 1 (PD-L1) [combined positive score (CPS) ≥10] as determined by an FDA-approved test.